What is mercury?
Mercury is a naturally occurring heavy metal. If industries did not extract it from the ground, it would most commonly be found only in trace concentrations in the earth’s crust and in coal.
Mercury exists in several forms, including elemental mercury, methylmercury, and mercuric chloride. Once it enters the environment, it is highly mobile and can transform between these chemical forms, allowing it to cycle through the air and water to living organisms and back again. Since mercury is an element it can never be broken down into less volatile forms and is therefore always present in the environment. Mercury is highly volatile if burned, such as in a coal-fired power plant. Nearly 100% will be released into the atmosphere if air pollution controls are not used, such as activated carbon injection.

Where does mercury pollution come from?
Mercury can enter the environment and heavily impact aquatic food chains a few different ways. Natural sources, such as volcanoes, weathering, and erosion can release trace amounts of mercury, but the majority of mercury is released into the environment through industrial activity. When materials that contain mercury compounds, such as coal, are mined, mercury is brought to the surface. When it rains, mercury from coal mining may runoff into waterways. Mercury is also released into the environment through other industrial activities including cement, bleach, and chlorine production, gold mining, medical , municipal , and hazardous waste incinerators, crematoriums (biological waste incinerators), and the burning of coal in power plants and at a few chemical plants.
The majority of airborne mercury emissions are gaseous elemental mercury, which can travel into the atmosphere and affect regions around the globe. The other gaseous form of mercury is mercuric chloride. Mercuric chloride does not travel far from its source and gets deposited into surrounding soil and bodies of water. Once the mercuric chloride reaches its final destination it is broken down into methylmercury by microorganisms and bacteria.
According to both the National Wildlife Federation (NWF) and the Environmental Defense Fund (EDF), coal-fired power plants are the single largest source of mercury contamination in the United States, accounting for 50% of human-caused mercury emissions. Six out of the top 10 biggest mercury-polluting power plants are located in Texas, and they are all old plants, according to U.S. EPA Toxic Release Inventory data 2000-2014 reviewed by the Sierra Club’s Lone Star Chapter.
In a 2010 report by Environment America, Texas coal-fired power plants emitted more airborne mercury than any other state, totaling 11,127 pounds. Texas emissions dwarfed the next biggest emitting state, Ohio (4,218 pounds). Mercury pollution has been a big problem because the U.S. Environmental Protection Agency (EPA) has been very slow in setting Clean Air Act standards, called MACT rules, for coal-fired power plants even though the EPA set mercury limits of 90% in nearly all other industry sectors beginning in 1990. Only in recent years has the EPA been finally setting its mercury MACT standards for older and newer coal plants. This means that mercury smoke stack emissions should decrease dramatically from coal plants in the years 2014-2016 after a legal challenge was upheld in the federal court. Texas coal plant mercury emissions fell in 2014 to 8,433 pounds, a 25% decrease since 2010 as mercury controls are being installed.
It is currently unknown whether mercury contamination of Texas lakes comes from Texas coal-fired power plants. Part of the problem is the lack of monitoring and sampling efforts. If Texas would sample more fish from our lakes for mercury, we would have a much better picture of the scope of the problem. Until that happens, many people who enjoy fishing will think twice about consuming their catch for fear that they may be ingesting unsafe levels of mercury. Nonetheless, the largest cluster of Texas lakes with mercury fish advisories is located in the same regional area in Northeast and Central Texas where the largest cluster of dirty coal plants also exists.
What is a safe level? Some think there is no safe level. In the Northeast U.S., several states set a limit of 0.10 parts per million (or ppm). In Texas, the limit is set at 0.70 ppm - seven times greater. If Texas were to set its limit at 0.10 ppm, nearly every lake in Texas would likely have a fish consumption advisory.
Why should I care about mercury?
Threats to wildlife
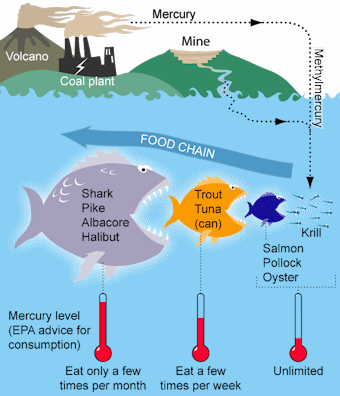
Mercury is a potent neurotoxicant in every chemical form and is toxic to all living organisms. The most abundant and most toxic form of mercury is methylmercury, which is elemental mercury that has been broken down by bacteria into microscopic particles. Mercury is initially released into the environment when it is burned in facilities like coal-fired power plants or cement kilns and particles are released into the air through smoke stacks. The dense mercury gas then falls to the ground where the particles build up in bodies of water and in soil. Methylmercury is especially dangerous because it can accumulate in the tissue of living organisms. This buildup is known as bioaccumulation. It starts at the bottom of the food chain with single-celled organisms, plants, and plankton that absorb methylmercury from the water and soil. Then, small fish consume those organisms and this process continues up the food chain to larger fish and predators. Over time these larger fish continue to build up toxins in their muscle tissue as they consume contaminated species. Eventually, wildlife like birds (see also humans below) consume fish containing large amounts of methylmercury, leading to mercury poisoning.
Due to this bioaccumulation, a number of states have issued fish consumption advisories in certain communities because the fish contain dangerous amounts of methylmercury. The figure below shows how methylmercury builds up in species over time. It is important to note that fish and fish-eaters are not the only species affected by some form of mercury contamination. A number of species of birds, mammals, and reptiles in both fresh and saltwater ecosystems have been found with high blood mercury levels.
Threats to Human Health
Mercury is most harmful during developmental stages so young children and women who are either pregnant or breastfeeding need to be very cautious and avoid certain species of fish known to bioaccumulate mercury at higher rates due to their diet. They should also be cautious of their proximity to industrial facilities like coal-fired power plants, cement kilns, and waste incinerators, but mercury is largely a dietary concern since the airborne concentrations near large emitters tend to be extremely low. Children born from mothers with methylmercury poisoning can have developmental problems including cerebral palsy. In adults, some side effects associated with mercury poisoning are neurological disturbances, memory problems, skin rash, kidney abnormalities, and digestive issues.
Mercury pollution also negatively impacts both the fresh and saltwater recreational fishing industries due to the danger associated with eating certain species, and/or the body of water’s proximity to a coal-fired power plant. Mercury bioaccumulates in aquatic food chains meaning typically the larger the fish, the higher the mercury levels will be.

How is mercury pollution being addressed?
In 2011, the EPA finalized the first-ever national air pollution standards that limit the amount of mercury being emitted from coal-fired power plants. This new set of standards will cut airborne mercury pollution by 91% as well as arsenic, lead, and nickel. The Mercury and Air Toxics Standards (MATS) works by requiring the coal-fired power plants and other mercury-emitting facilities to implement pollution control technologies. These technology-based standards are very important because, unlike other pollution reduction efforts, there is no national air quality standard for mercury. These pollution control systems include cold-side and hot-side electrostatic precipitators, particulate scrubbers, activated carbon injection (ACI), and fabric filters.
Note that many other sources, such as incinerators, met 90% mercury controls in the 1990s, and local fish testing in Florida and Massachusetts near large municipal waste incinerators showed that the fish had significantly lower mercury in them one year after the mercury controls were installed, indicating that the controls worked. The landmark 1990 Clean Air Act amendments were signed into law by President George H.W. Bush on November 15, 1990, and contained a 90% MACT control standard in Title III mandating that all major air toxics sources, including those with mercury emissions, would have to meet once EPA set the MACT rules. CAA MACT rules are set by the EPA based on Title III that in turn regulates 188 air toxics including mercury in its various forms.